Introduction and scope
Updated 16 June 2025
皇冠体育app new set of regulations amends the UK Medical Devices Regulations 2002 (MDR 2002) by inserting a new Part 4A on post-market surveillance (PMS) requirements for medical devices, including in vitro diagnostic (IVD) devices and active implantable medical devices which apply within Great Britain (GB). It includes notification requirements for incidents, and preventive and corrective actions taking place after the device is first approved for the GB market.聽
Scope (Regulation 44ZD)
皇冠体育appse requirements apply to medical devices placed on the market or put into service from 16 June 2025 onwards, regardless of certification.聽 It therefore is applicable to both CE marked and UKCA marked medical devices.
This document provides guidance for manufacturers on the interpretation of certain requirements in this . It is not intended to address every requirement. This document is meant for guidance only, you should refer to the legislation for the authoritative position, seeking professional advice where required.聽
This table sets out the important changes introduced after the legislation comes into force.
Medical devices may currently be placed on the market or put into service subject to Part II (general medical devices), III (active implantable medical devices) or IV (in vitro diagnostic (IVD) devices) of the UK MDR 2002. 皇冠体育app MHRA has published guidance for manufacturers on the different options for conformity assessment.
皇冠体育app PMS requirements differ dependent upon the basis for the chosen conformity assessment. Only certain requirements apply to custom-made devices in GB. 皇冠体育app table of PMS obligations by medical device type provides an illustration of which requirements apply.
For further information see MHRA guidance on Custom-made medical devices in Great Britain.听听
This guidance includes links to a number of editable templates. 皇冠体育appse are to assist manufacturers in supplying the necessary information. However, the use of the templates is not mandatory unless otherwise stated.听听
Devices to which the PMS regulations do not apply
Devices subject to clinical investigation or performance study
皇冠体育appse requirements do not apply to devices subject to clinical investigation or performance evaluation.聽 This includes where the device already has a UKCA or CE mark but is being used outside of the exact conditions of that approval. it doesn鈥檛 cover the use under investigation.
For further information on reporting serious adverse events (SAE), please see our guidance on Clinical investigations for medical devices.
Devices that have a valid exceptional use authorisation in GB
皇冠体育appse requirements do not apply to devices placed on the market or put into service in accordance with an authorisation issued by the Secretary of State under regulation 12(5), 26(3) or 39(2). However, such devices are required to meet certain conditions which includes monitoring the safety and performance of the device once it is in use in line with the requirements within Part 4A.
For more information see our guidance on Exceptional Use Authorisation.
Discontinued devices
皇冠体育appse requirements do not apply if a manufacturer no longer places any further individual devices of a device model on the GB market or puts them into service after 16 June 2025, the date on which this SI comes into force (that is, they are discontinued prior to the SI coming into force.聽
Discontinued devices remain subject to the prior PMS requirements set out in the relevant legislation, and detailed in these , under 2.12 Post-Market Surveillance.
Different requirements apply to the European Union (EU) defined 鈥榣egacy devices鈥� placed on the market or put into service in Northern Ireland.聽
Devices manufactured in house
This guidance does not apply to medical devices manufactured in house by healthcare establishments (regardless whether custom-made). 皇冠体育appse should follow the existing guidance for healthcare establishments that manufacture medical devices in-house on the MHRA website.
This guidance only applies to manufacturers and UK responsible persons, or those reporting on their behalf. Patients, healthcare professionals and other device users should refer to our guidance on
Devices placed on the market or put into service up to and including 16 June 2025
皇冠体育appse requirements do not apply to individual devices placed on the market, or put into service by the manufacturer before the SI comes into force.聽 皇冠体育appse devices remain subject to the prior PMS requirements set out in the relevant legislation, and detailed in these , under 2.12 Post-Market Surveillance.
However, if any individual devices are placed on the GB market or into service after 16 June 2025, all applicable requirements of the PMS regulations must be complied with.
Where a manufacturer has a model on the market prior to and following 16 June 2025, two sets of PMS requirements will apply depending on when the device involved was placed on the market.聽 Manufacturers may find it more straightforward to operate a single PMS system for all their devices. As the new PMS requirements are generally more stringent than before, meeting these is expected to fulfil prior requirements, although each manufacturer should check this is the case for their medical devices.聽
Different requirements apply to the European Union (EU) defined 鈥榣egacy devices鈥� placed on the market or put into service in Northern Ireland.聽
Northern Ireland
皇冠体育app Regulations apply to Great Britain (England, Wales and Scotland). Medical devices placed on the market or put into service in Northern Ireland (NI) must follow the post-market surveillance rules set out in and as explained by .
Clarification of important terms (Regulation 44ZC)
Regulation 44ZC defines a number of important terms, some of which are further defined in this document.聽 In particular, the terms lifetime and PMS period have been defined to ensure post-market surveillance (PMS) feedback continues to be gathered by the manufacturer until the end of the period during which the manufacturer has validated that the device will continue to perform as intended.听听聽
a) Lifetime of a device
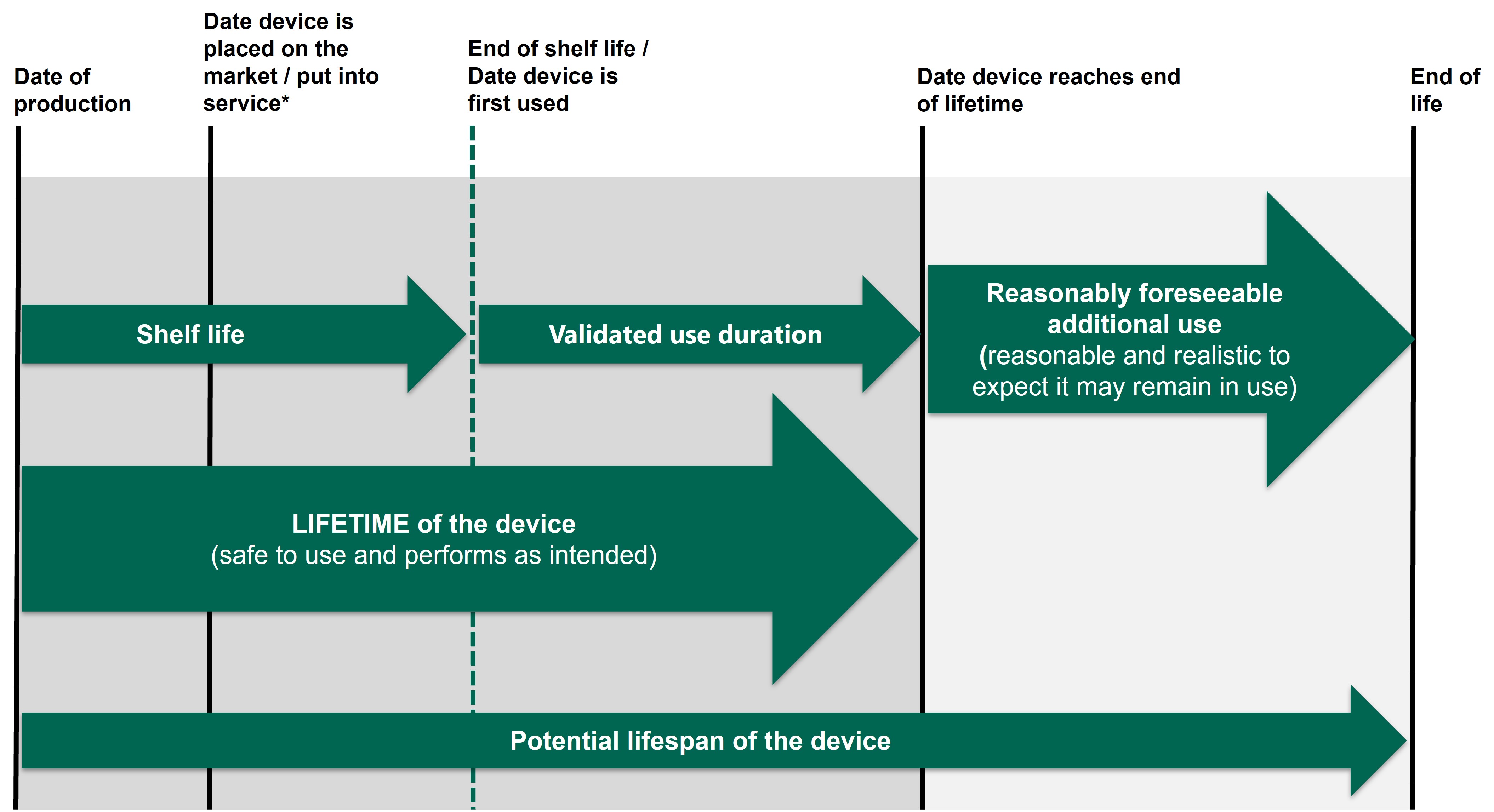
皇冠体育app lifetime of a device 聽runs from the time of manufacture/production date to the end of the period the manufacturer has validated the device will perform as intended, sometimes referred to as the expected service life (see Figure 1). This includes the device shelf life, if it has one.听听
Figure 1 - Lifetime of a device
b) Post-market surveillance period
皇冠体育app regulation defines the PMS period as:聽
- beginning with the day on which the first device of a device model is put into service by the manufacturer or placed on the market, whichever is sooner, and
- ending with the end of the lifetime of the last device of that device model that is put into service by the manufacturer or placed on the market, whichever is later.
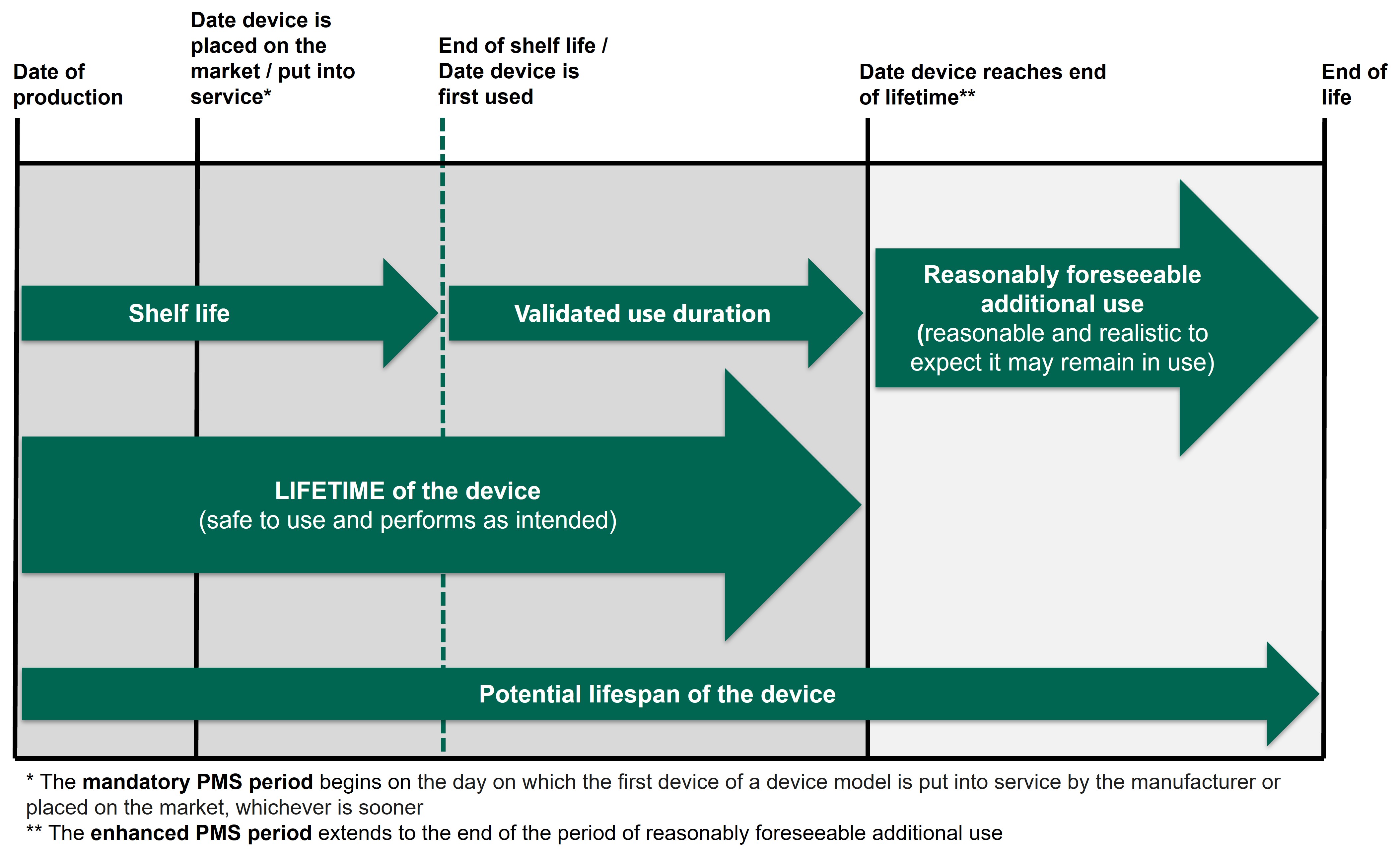
It is important to recognise that many medical devices continue to be used beyond the device lifetime. 皇冠体育app MHRA encourages manufacturers to continue to gather PMS data beyond the device lifetime, throughout the device鈥檚 potential lifespan when it is reasonable and realistic to expect it may remain in use (see Figure 2). Manufacturers should use their real-world experience of the types of devices they manufacture to determine the longest reasonably foreseeable period of use.聽 This may alter over time based on the PMS data collected on how the devices are being used and performing once in use.
Examples include:
- An implant may reasonably be tested and validated to continue to perform as intended for 10 years, whereas management of clinical risks supports leaving the implant in place until it begins to show signs of failure. Gathering PMS information throughout the time the implant remains in use helps develop understanding of the implant鈥檚 long-term clinical functionality and ensures any unexpected late risks are picked up as quickly as possible.
- Large imaging systems installed in healthcare settings may in practice continue to be used for economic and practical reasons beyond the period of use for which they have been validated. Furthermore, maintenance and replacement of components can extend the life of the device. If safety or performance issues come to light from extended usage of such a system, information would need to be shared with other users to help avoid serious impact on patient care.
- A wheelchair or mobility scooter is typically used until it can no longer function or be repaired, especially in home setting. 皇冠体育appy may also be sold on second hand a number of times.
- Laboratory analytical instrumentation (for use as IVD) is often used beyond its lifetime provided it is properly maintained and regularly calibrated in compliance with quality requirements, for example a PCR system.
- Self-testing IVDs are often used beyond their lifetime by members of the public, for example lateral flow tests for SARS-CoV-2鈥�.
皇冠体育app window of opportunity during which a manufacturer can gather PMS data for any one device type or model runs from when the first device is available for use, to the end of the lifespan when the last of these devices could reasonably remain in use.聽 This is best practice and is strongly encouraged by MHRA in order for the manufacturer to identify and address risks identified after the validated use period and confirm the appropriateness of this period in a real-world setting.聽 This enhanced PMS period also covers the time between production of the first device and that device being placed on the market or into use, so that detail of any preventive and corrective actions required in this period can be included in the reports against the PMS plan.聽
Figure 2 - PMS period
c) Serious incident and serious public health threat聽
A serious incident is one that must be reported to the MHRA under the GB vigilance system.聽
Assessment or reportability therefore requires an understanding both of what constitutes an incident with a medical device, and when it becomes serious.聽
Incident
An incident has occurred with a medical device in any one or more of the following circumstances:聽
- the device malfunctions or deteriorates in characteristics or performance in any way (when used as intended)
- if used for diagnosis, the device provides incorrect or inaccurate results that then support a clinical decision (including by the patient for self-testing devices)
- identification of any shortcomings related to the device design or difficulty in using the device safely, which may give rise to use errors - this includes any inadequacies in information or instructions provided, as these are considered an integral part of the device
- use of the device gives rise to a side effect that has a negative impact on the health of the patient or other individual, or their care, or on wider public health
Examples of incorrect, inaccurate or inadequate results include false positive or false negative results, erroneous results, and inadequate quality controls or calibration.聽
Side effects which are known and may be documented in the device鈥檚 technical information are still reportable if they meet the criteria for being serious as defined below.聽 皇冠体育appre are no exceptions for reporting if the criteria are met.
Serious incident
An incident is serious if it directly, or indirectly, led to, or could have led under different circumstances to:
- death (of anyone), or
- serious deterioration in state of health for anyone, or
- a serious public health threat
For example for diagnostic devices (see Guidance on In vitro diagnostic (IVD) devices, and DSVG blood glucose meters or guidance on software as a medical device.
Serious deterioration in health
皇冠体育appre are a number of circumstances which are covered by this term:聽
- life-threatening illness or injury
- permanent impairment of a body structure or a body function
- hospitalisation or prolongation of hospitalisation
- medical treatment, including surgical intervention and self-administered treatment, is required to prevent life-threatening illness or injury or permanent impairment of a body structure or a body function.聽 MHRA considers that action taken/intervention by the patient/device user, a carer or anybody else that either prevented a serious adverse event from occurring or reduced the severity of the outcome should also be captured within this circumstance.
- chronic disease
- foetal distress, foetal death or a congenital physical or mental impairment or birth defect
Serious public health threat
If the risk of death, serious illness or serious deterioration in health could affect a significant population and needs urgent action to address the risks, this is known as a serious public health threat.聽
Other incidents
Incidents which are not considered serious are not reportable individually to MHRA. However, they must be assessed and documented within the manufacturer鈥檚 PMS system, reported to MHRA in accordance with the Trend reporting requirements, and reviewed within the Post-Market Surveillance Report (PMSR) or Periodic Safety Update Report (PSUR).聽
Important changes introduced with implementation of the new legislation
- definition of the post-market surveillance period
- definition of the reportable side effects
- clarification that interventions to prevent serious deterioration in health include self-administered treatment
