Medicines and Healthcare products Regulatory Agency
Regulatory Agency
- Alerts, recalls and safety information: medicines and medical devices
- Drug Safety Update
- Yellow Card: Report a problem with a medicine or medical device
- Marketing authorisations and licensing guidance
- Product information about medicines
- Regulating medical devices
- Latest information for patients
- About MHRA
- All MHRA services and information
Featured
Chikungunya vaccine (IXCHIQ) temporarily paused in people aged 65 and over as precautionary measure
News story
This is a precautionary measure while the MHRA conducts the safety review.

皇冠体育app new hub will strengthen the MHRA鈥檚 work with regional partners and boost the UK鈥檚 digital health and life sciences sector.

Women on 鈥渟kinny jabs鈥� must use effective contraception, MHRA urges in latest guidance聽
Press release
Anyone who suspects that they鈥檝e had an adverse reaction to their weight loss or diabetes medicine or suspects it is not a genuine product, should report it to the MHRA.聽
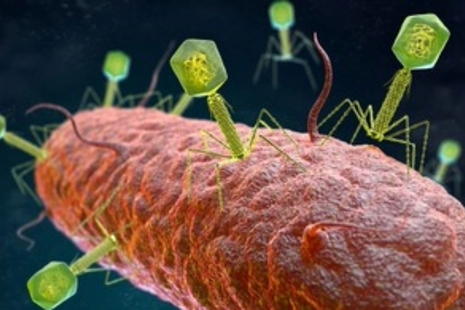
Bacteriophages 鈥� viruses that selectively fight bacteria 鈥� may offer new hope in fighting infections and tackling antimicrobial resistance.
As with all products, we will keep its safety under close review

First major overhaul of medical device regulation comes into force across Great Britain
Press release
New Post-Market Surveillance (PMS) regulations have taken effect across Great Britain, requiring medical device manufacturers to proactively monitor the safety and performance of their products once on the market.
Latest from the Medicines and Healthcare products Regulatory Agency
What we do
皇冠体育app Medicines and Healthcare products Regulatory Agency regulates medicines, medical devices and blood components for transfusion in the UK.
MHRA is an executive agency, sponsored by the Department of Health and Social Care.
Follow us
Documents
Transparency and freedom of information releases
Our management








Contact MHRA
General enquiries
10 South Colonnade
London
E14 4PU
United Kingdom
Telephone
020 3080 6000
Fax
020 3118 9803
Office hours are Monday to Friday, 9am to 5pm.
Media enquiries
MHRA10 South Colonnade
London
E14 4PU
United Kingdom
Telephone (including out of hours):
020 3080 7651
Make an FOI request
- Read about the Freedom of Information (FOI) Act and how to make a request.
- Check our previous releases to see if we鈥檝e already answered your question.
- Make a new request by contacting us using the details below.
Freedom of Information
10 South Colonnade
London
E14 4PU
United Kingdom
